Feb 23, 2021 | Indian Patents Act 1970
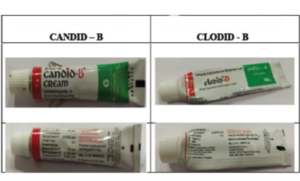 In this case of trademark infringement, an exemplary amount of Rs. 1.5 crore was awarded by the Hon’ble Bombay High Court in favour of Glenmark Pharmaceuticals Ltd. (hereinafter referred to as ‘Glenmark’). Galpha Laboratories Ltd. (hereinafter referred to as Galpha) was found to be ‘habitual offender’ for trademark and copyright infringement. What makes this case interesting is, firstly the Galpha’s decision to not contend the suit and secondly the exemplary damages awarded by Hon’ble Bombay High Court. Brief Facts of the case are as follows:
In this case of trademark infringement, an exemplary amount of Rs. 1.5 crore was awarded by the Hon’ble Bombay High Court in favour of Glenmark Pharmaceuticals Ltd. (hereinafter referred to as ‘Glenmark’). Galpha Laboratories Ltd. (hereinafter referred to as Galpha) was found to be ‘habitual offender’ for trademark and copyright infringement. What makes this case interesting is, firstly the Galpha’s decision to not contend the suit and secondly the exemplary damages awarded by Hon’ble Bombay High Court. Brief Facts of the case are as follows:
- Galpha’s mark “CLODID-D” was alleged to be infringing packaging and trade dress of Glenmark’s mark “CANDID-B”. It was further alleged that Galpha has copied word mark, art-work, colour scheme, font style, manner of writing, trade dress of the Glenmark’s product “CANDID-B”.
- With regards to Curetech Skincare, the position was made clear that they were only the contract manufacturer of the product of Galpha. The copy of contract manufacturing agreement was also supplied by Curetech according to which art-work, labels and marks were provided by the Galpha, and they are not claiming any right over the disputed mark.
- The main dispute pertaining to infringement of trademark of Glenmark by Galpha was contended before the court.
Arguments
By Glenmark i.e., Plaintiff:
- That this is not the first time Galpha is infringing their trademarks, an earlier cease and desist notice was cited by Glenmark, wherein Galpha was asked to stop infringing upon the trademark of Glenmark, “ASCODIL”, by using deceptively similar mark “ASCORIL”, to which an unconditional apology and undertaking was provided by Galpha.
- That apart from copying Glenmark’s trademark, Galpha has copied marks of various other pharmaceutical companies for which suits are pending. Further in the case of Win-Medicare Pvt. Ltd. Vs. Galpha Laboratories Ltd. & Ors {2016 (65) PTC 506 (Del)}, Delhi High Court has observed that Galpha is a ‘habitual offender’.
- That apart from infringement, there are several other instances wherein medical products of Galpha were found to be “Not of standard quality/Spurious” by Central Drugs Standard Control Organization, various articles were cited wherein it was revealed that Galpha has violated standards set out by Food and Drug Administration (FDA).
- That on basis of such repeated infringement and violation of standards a stricter punishment has to be awarded upon Galpha to deter them from any such illicit acts in future
By Galpha i.e., Defendant:
- Galpha, surprisingly, admitted to all the allegations put forth by Glenmark and contented that they should have been more precautious and diligent before adopting and using the trademark and that they are willing to submit themselves to the decree of court.
Issue
Whether Galpha by use of mark “CLODID-B” is infringing upon mark of Glenmark, “CANDID-B”?
Judgement
The marks in dispute were presented before the court and the court took notice that the mark of Galpha, “COLDID-B” is nothing but a direct copy of mark of Glenmark, “CANDID-B”. It was observed by court that “Drugs are not sweets. Pharmaceutical companies which provide medicines for health of the consumers have a special duty of care towards them. These companies, in fact, have a greater responsibility towards the general public”. Strict approach was followed by Bombay High Court, and based on the evidences and documents produced by Glenmark, the court observed that there is no doubt that Galpha is a habitual offender with a set mode of operation of copying brands of other to make profits.
Further it was observed that Galpha has copied trade dress, colour scheme, art-work, font style and even manner of writing of Glenmark’s product, and keeping in mind the strictness to be followed in medical products; habitual disregard by Galpha to rights of others; and keeping public interest at priority, Hon’ble High Court of Bombay imposed Rs. 1,50,00,000/- as the exemplary costs on the Galpha.
Thus, by the documents and evidences produced before the court, it was observed that Galpha is not only a habitual infringer but also habitual offender of law, medicine being directly related to public health needs more scrutiny and hence, Galpha cannot escape its liability by pleading that they accept all the charges and submit themselves to the suit. They have to be responsible for their actions and Rs. 1.5 crore was awarded as an exemplary damage to deter Galpha from any infringement and violation in future. Court while passing such judgement relied not only on Trade Marks Act but also on general principle of law.
By: Dhruv Dangayach, Ramaiah College of Law
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join Linkedin Group: Innovation & IPR
Whatsapp: +91 74838 06607
Feb 22, 2021 | Indian Patents Act 1970
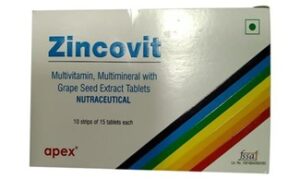 Apex laboratories, herein is the manufacturer of pharmaceutical products and on 16th March 1988 it adopted the trademark “ZINCOVIT” for their product, subsequently, it obtained the registration and has been using the said mark for its products.
Apex laboratories, herein is the manufacturer of pharmaceutical products and on 16th March 1988 it adopted the trademark “ZINCOVIT” for their product, subsequently, it obtained the registration and has been using the said mark for its products.
In February 2006 Apex Laboratories came to know about the mark of Zuventus Healthcare i.e., “ZINCONIA”, which has been used for manufacture and sale of similar product as that of Apex Laboratories. For the same infringement case was brough, and an ex-parte injunction was subsequently obtained. The said decision was challenged before Delhi High Court by Zuventus, wherein the ex-parte injunction order was vacated by Single Judge holding that there is no similarity between the mark “ZINCOVIT” and “ZINCONIA” and that the word ‘zinc’ is common name in trade. Appeal to such order was made by Apex Laboratories which was the subject matter of this case.
Arguments
By Apex Laboratories i.e., Appellant:
That the mark of Zuventus, “ZINCONIA”, is deceptively similar to the mark of Apex, Apex is the registered proprietor of the mark “ZINCOVIT” and has been extensively using the mark since registration, thus injunction is ought to be given in favour of Apex Laboratories.
By Zuventus Health Care i.e., Respondent:
That the Apex laboratories is not the exclusive proprietor of the mark “ZINCO”, there are several other registered owner of the mark “ZINCO”, moreover mark of Apex, “ZINCOVIT” and mark of Zuventus, “ZINCONIA”, are not visually and phonetically similar and the essential features in both the marks i.e., colour combination, scheme of writing are entirely different.
Issue
Whether the mark of Zuventus Healthcare Ltd. i.e., “ZINCONIA” is deceptively similar to mark of Apex Laboratories i.e., “ZINCOVIT”?
Judgement
Various precedents were cited before Hon’ble Delhi High Court by the counsel of both the parties, taking them into consideration it was held by the court that the medicine of Apex Laboratories and Zuventus, contains common element zinc; therefore, the word ‘zinc’ is common to trade and public juris. Apex Laboratories cannot claim exclusive use over the word ‘zinc’. It was also observed that while comparing for infringement a mark has to be taken as whole, it would not be right to spilt the mark for purpose of injunction just because they contain the word ‘zinc’ and Apex cannot claim exclusivity by using such mark.
Thus, it was held that the marks are not visually and phonetically similar so as to cause confusion and the order of Single Judge for vacating the order of interim injunction was upheld.
By: Dhruv Dangayach, Ramaiah College of Law
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join Linkedin Group: Innovation & IPR
Whatsapp: +91 74838 06607
Feb 16, 2021 | Contracts
A Software Development Contract is a contract between a corporation and a software developer in which the firm expresses its concepts and requirements. The developer then proceeds to design the software according to the company’s schedule constraints.
Such agreements are common in software organizations where developers are contracted to create computer software for both commercial and private use. As a result, it’s critical to determine the parameters of both developers’ and companies’ rights in relation to software.
 A software development agreement is a contract in which one party (the Developer) undertakes to develop software for another party (the Client or the Company). While design and development procedures differ based on the complexity of the project and the team employed, there are a few important issues that are universally applicable and should be taken into account while negotiating the contract.
A software development agreement is a contract in which one party (the Developer) undertakes to develop software for another party (the Client or the Company). While design and development procedures differ based on the complexity of the project and the team employed, there are a few important issues that are universally applicable and should be taken into account while negotiating the contract.
A written development agreement can be thought of as a road map. It will prevent disagreements if it is effectively drafted. It will provide solutions to problems if they arise. If the parties get into a disagreement, it will determine their legal obligations to each other. To create a software development contract, what is necessary is an understanding of the potential complexities. This article outlines the most crucial aspects of a software development agreement.
What are the Ingredients of a Software Development Contract? The following information is commonly engraved in a software development contract:
Duties of the developer
All of the developer’s responsibilities must be outlined in the contract, including:
- Software development in accordance with the company’s Software Requirements Specifications and in accordance with the company’s milestones at various stages.
- Answering concerns regarding the program for a specified period of time following delivery.
- The developer must also acknowledge the company’s right to terminate the contract if the developer violates the terms and conditions of the contract.
- The limit of support to be offered by the company and the time period for completion of the project.
Services to be offered
The contract should define the development services expected from the partner. This section of the contract usually relates to the contract’s specification, which is an inherent aspect of it. The project scope that is to be delivered should be clearly stated in the specification. Try to issue the specifications in as much detail as possible to safeguard both the parties from conflicts.
The mechanism for changing the scope should also be described in the Services section. Any changes recommended by any party should be made in writing, in accordance to good practice. The addition of a statement indicating that any change request must include the following information:
- Description of the change
- The impact of the modification on the project’s cost and timeline.
Acceptance
The acceptance period after delivery may be specified, during which the company can assess and test the software to ensure that it is completely compliant with the specifications. When the developer provides a product that meets all of the specifications, the delivery is considered complete. A Rejection clause could be introduced, allowing the corporation to reject the product if the software does not meet their specifications or if the developer is unable to deliver the complete product. The refusal must be written down.
Acceptance testing
Specify whether acceptance testing will be performed on the developer’s end or by the vendor. Acceptance testing is typically performed at the conclusion of each development phase, so this section may also refer to the detailed project plan.
The contract should state in the context of acceptance testing:
- Who conducts the tests?
- The length of time it takes to complete the tests
- The manner in which test findings are communicated (the best way is to notify the other side in writing about the acceptance)
Specifics on delivery
The contract must state the date by which the software must be completely functional and correspond to the standards, as well as:
- The developer’s corrections in the event of non-conformity in certain areas of the finished product. And,
- The manner by which the company will notify the developer if any non-conformity is discovered.
Training
The developer’s obligations for training the corporate personnel in the usage of the software, as well as the approximate hours of training and the location of training, must be specified. Any additional expenditure associated with training must also be disclosed.
Maintenance and Support
The duration of the developer’s support and maintenance for the software program, as well as the terms of renewal, must be specified.
Maintenance
As new technology arrives, software evolves at a breakneck pace. This implies that if the software which is commissioned for has to work with other programs, it may need to be updated or upgraded before utilizing it. To avoid this issue, the software development agreement should include specific information on:
- What upgrades are expected to be required in order to keep up with technological advancements?
- Will the Company’s requirement for additional functionality be met through a maintenance contract or existing support services obligations?
- Who will be in charge of updating and upgrading the system?
It’s also worth noting that most developers reserve the right to stop providing support and maintenance for any previous version of software if a newer version is made accessible to the Company (the client).
Support
Although some support provisions may be included in a software development agreement, it is more common for support services beyond the installation and testing phase to be charged separately, or to be quoted separately if acquired as an Annual Support Agreement.
If intended to add assistance provisions in the contract, it should be specified:
- How will assistance be provided? (email, telephone, or in person).
- The kind of issues that the support services will address.
- Response times, particularly with regard to an Annual Support Agreement.
- Any limitations on support services, as well as any services that may be subject to additional fees.
Project time and cost
This component establishes the agreement on the project’s completion schedule and cost. Indicate the hourly rates, development phases, goals, and deadlines for each.
The agreement should also state who is responsible for the delays by both parties. The inclusion of any partial payments that have been agreed upon based on the development progress. Annexes to the contract, such as payment schedules or development plans, may be referred to. The both parties must mandatorily sign any supplemental documents.
Compensation
The contract must state the total monetary consideration payable by the corporation to the developer, as well as the breakup, which is commonly stated on an hourly basis. Further, the developer must specify the time intervals during which recurring bills must be sent. Any details concerning the initial and subsequent payments must also be mentioned.
Intellectual Property Rights
The contract should state that the company will be granted copyrights and other intellectual property rights in relation to the software, such as trademarks if applicable.
One of the most crucial sections of the software development contract is this one. The company owns the software created as a result of the project, which should be stated clearly in the contract. Make certain that the contract has the following provisions:
- The source code should be owned by the company. By obtaining ownership of the source code, the company gains the ability to use or alter as it may see fit.
- In the event of contract termination, the company immediately owns the code has been completed thus far.
- All resources developed throughout the development process, including wireframes, drawings, and blueprints, must be destroyed.
The development business, on the other hand, can only reassign the rights to the property that they built. Any open-source tools that were utilized will be made available to the public.
Further Changes
All information regarding the procedure for requesting changes to the software’s specifications must be provided. If the adjustments are appropriate, the developer may be required to accommodate them at no additional expense to the company. The corporation may also agree to provide additional pay in the future if major adjustments are implemented. It’s also possible to include provisions for reversing such changes.
Confidentiality
Confidentiality is one of the most important facets of a software development contract. The developer agrees not to provide any information about the company, its activities, or its clients to any third parties. In addition, the developer commits not to make copies of the software or distribute it to third parties.
While the Confidentiality part is standard in most service contracts, it is absolutely crucial in the information technology industry. Specify the information that is regarded confidential and the responsibility for the said disclosure.
The confidentiality terms in most software development contracts survive the contract itself, which means that the confidentiality should be maintained even after the contract is completed.
Warranties from the developer
(a) The developer must guarantee that the program does not violate any agreements the developer has with third parties, and that it does not infringe on any third party’s intellectual property rights.
(b) The developer warrants that the program will run smoothly according to the company’s expectations and agrees to resolve any bugs that arise within the stated time frame. As the creator of the intellectual property, he or she must assign the rights to the corporation under the terms of the agreement.
(c) A disclaimer stating the developer is not responsible for any warranties not expressly stated in the contract may also be included.
Assignment
It should be mentioned that the developer may not delegate any contract rights to a third party without the company’s consent.
Indemnification
The developer undertakes to indemnify the company from any lawsuits that may occur as a result of the Developer’s infringement of third-party intellectual property rights.
No changes are permitted unless they are made in writing.
It should be mentioned that no amendment of the agreement will be permitted unless both parties agree.
Acknowledgements
The developer is an independent contractor, and the contract does not constitute any partnership, joint venture, employer-employee connection, or principal-agent relationship between the company and the developer.
It should also be noted that the contractual relationship will be one of “work for hire,” as defined by the Copyright Act of 1957.
Termination
The contract may specify the methods for terminating the agreement. It could be for particular reasons specified in the contract after giving the other party sufficient notice, such as a material breach of the contract’s terms or failing to perform any part of the contract. The non-breaching party has sole discretion over whether or not to cancel the agreement.
Jurisdiction in Cases of Dispute
The contract must state which court will have jurisdiction over any dispute arising from a breach of any of the provisions.
In the complicated realm of Intellectual Property Rights, a well-drafted Software Development Contract protects both the company’s and the developer’s rights and eliminates ambiguity.
Author: Vinita Gaud, Pravin Gandhi College of Law
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join LinkedIn Group: Innovation & IPR
WhatsApp: +91 74838 06607
Nov 14, 2020 | General, Indian Patents Act 1970, Patent
The role of a patent agent has been a source of curiosity since enactment of the Patents Act 1970 (the Act). The provisions relating the patent agents have been comprehensively amended in 2002 amendment enforced from 20th May 2003. One of the most commonly asked questions is whether it is important to be patent agent especially when there is huge scope for people who are expert in patent searches, who may or may not be patent agent. In this article, lets analysis, who can be a patent agent, what are advantages of the same and how are the job prospects better if one is a patent agent?
Patent specification required to filed for obtaining a patent is invariable termed as a techno-legal document as it is a combination of technical description and the claim, which are purely legal in nature. The patent specification discloses technical details of the invention and defines scope of the invention by restricting legal rights to the claims. Since by nature, a patent is a technical document, in order to draft patent specifications, one needs excellent writing skills and expertise as well we deep understanding of the subject matter and knowledge about the patent law. For a person to work in the area of patent law, he has to have cocktail of all these attributes.
Who is a patent agent?
Technically/legally speaking the patent agent is a person so registered under the Patent Act. However, in practice the Patent Agent is a person, which is the link between the inventor and the patent authorities, such as, the Controller, who facilitates the work of grant of patent by assisting the inventor, the Controller or his subordinate officials. He has exclusive right to do certain acts in the process for obtaining a patent and has exclusive right to practice before the Controller. The Patent Agent is also allowed to appear before patent office of other PCT member country in respect of national phase of the corresponding application.
The patent agent should have thorough knowledge of the Patent Act and rules, Patent Co-operation Treaty provision and prosecution therein and also comparative knowledge of procedures in other important countries such as US, EP, Japan and China.
Who can become a patent agent?
A person for being eligible to register himself in the register of the Patent Agents under the Act must have qualification prescribed under section 126. These qualifications are as follows:
- The person shall be a Citizen of India;
- He must have completed 21 years of age;
- He shall possess a Degree in Science, engineering or technology from recognized university or other equivalent qualification as prescribed by the Government; and
- Should have passed the qualifying examination conducted by the Patent Office or Should have worked as examiner or discharged functions of Controller for not less than ten years.
Additionally, he also must have paid such fee as prescribed. The Act does not define the degree in science, technology or engineering, hence, these terms are open to interpretation. The equivalence of qualification should be as per notification made by the Government in this regard. For example there are graduations awarded by Universities in certain subjects like Mathematics, Statistics, Geography in both Science and Arts. The Diploma Holders in engineering or Bachelors’ degree in engineering from a foreign university, who are equally knowledgeable as graduates are not allowed to appear. Their case needs to be considered sympathetically by the Government. Prior to 2002 amendment, there was no such restriction. The restriction though well intended must also take into consideration of various diploma holders in science, technology and engineering who may be equally learned in science, technology and engineering but might not get opportunity to be a bachelor for various socio-economic reasons.
“However, one must understand that being a patent agent is not everything. There are several proceedings under the Act which take place in the courts and being an Advocate is always of great advantage. Even if one does not wish to practice in courts, in addition to patent law, sound knowledge of law of interpretation, contracts, Indian Constitution is of great value to attain better hold on the subject”.
Those Agents who are already registered before the amendment shall subject to payment of renewal fees continue to be registered so irrespective of their qualifications. Before 2002 amendment came into force any Advocate under the Advocate’s Act could register himself as a patent agent, without appearing for examination, however after the amendment came in to force on 20th May 2003, even an Advocate also needs to be a science, and engineering or technology graduate and is required to appear for the patent agent examination conducted by the Patent Office.
What are the advantages of becoming patent agent?
There are many advantages of being a patent agent, more so after the 2002 amendment to the Act. Prior to 2002 amendment, Section 132(a), nothing in the chapter XXI relating Patent Agents prohibited any person not being a Patent Agent who was duly authorised by the applicant from drafting any specification or appearing before the Controller and an Advocate from taking part in any proceeding under the Act except drafting specification. The situation has dramatically changed after the 2002 amendment. Now the Patent Agents only have exclusive right to practice before the Controller as spelt out in Section 129(2). Except the applicant himself, even advocate cannot appear in general but can appear on behalf of the party in proceeding under the Act only if the party is also taking part in the said proceedings. Thus the role of the patent agent in the patent prosecution has been significantly enhanced by the said amendment in 2002. Only patent agents can prepare all documents, transact all business and discharge such other function as prescribed under the Act and the role of other authorised persons and advocates has been considerably limited.
A registered patent agent also gets added weightage and advantage over others in securing jobs if he/she does not want to practice independently. The Advocate firms will employ them as they only can appear before the controller for prosecuting the patent applications filed by the firm. The registered patent can also practice before the IPAB even if he is not an Advocate. It is interesting to note that a registered patent agent can also act as an agent for design registration under the Designs Act 2000.
However, one must understand that being a patent agent is not everything. There are several proceeding under the Act which take place in the courts and being an Advocate is always of great advantage. Even if one does not wish to practice in courts, in addition to patent law, sound knowledge of law of interpretation, contracts, Indian Constitution is of great value to attain better hold on the subject.
Patent Agent Examination
The qualifying patent agent examination is conducted by the office of the Controller. The particulars of the examination, the curriculum and qualifying marks are given in Rule 110. Upon passing the examination, the successful candidates are required to follow a registration process as provided in chapter XV of Patent Rules.
The examination consists of two written exams and viva. Paper 1 relates to Patent Act and Rules, Paper II relates to drafting and interpretation of patent specifications and other documents. Each written paper carries maximum 100 marks. The Viva carries 50 marks.
The Rule 110 (3) regarding qualifying marks has been amended after the decision of the Delhi High Court in Anvita Singh V/s Union of India and Others in 2012 and Renu Bala case. The amended rule 110(3) reads as follows:
110(3) A candidate shall be required to secure a minimum of fifty marks in paper I and paper II and shall be declared to have passed the examination only if he obtains an aggregate of sixty percent of total marks.
The amended rule has practically reduced the viva redundant as one need to only have compulsory appearance in the viva. If a candidate secures 150 marks in both the written papers and only appears for viva.
The detailed nature of the paper I and paper II is as follows:
Paper 1: Total 100 Marks
It is divided into part A1, A2 and B.
Part A1 (30 Marks)
- 15 multiple choice questions Each question carries two marks;
- Candidate to answer all the questions in this section; and
- To choose the right answers from maximum six choices and maximum two correct choices.
Part A2 (10 marks)
- True or false, 10 questions one mark each.
Part B (60 Marks)
- 8 Subjective type questions. Candidate to answer any 6 questions.
Paper 2: Total 100 Marks
It is divided into part A, B1 and B2.
Part A (40 Marks)
- Consist of 6 questions of 10 marks each and the candidate to attempt any 4 questions. The questions will relate to drafting and interpretation of patent specifications and other documents
Part B (60 marks)
It consist of parts B1 and B2.
- Part B1 is compulsory and will consist of 1 question relating to drafting of claims and abstract from a given description of an invention.
- Part B2 consists of 2 questions and the candidates will be required to attempt any 1 question. Out of the two questions, one question will relate to general engineering and the other question will relate to field of chemistry/life sciences.
The prospecting patent agents may refer to old papers available on the patent office website to understand nature of questions asked in the examination.
Job opportunities for a patent agent
A patent agent, being an expert in patent law as well as technology shall have good opportunities not only in IP department of any R&D oriented firm but also the law firm. Areas of work could be patent specification drafting, filing, prosecution and performing patent searches of various kinds. However, clearing the patent agent exam and registration as a patent agent alone is no more than a certification. In the super specialized area like patents, one need to work really hard and acquire skills and expertise for long term and sustainable career growth. Career of a patent agent can always extend to more specialized areas, such as, patent valuation, technology commercialization, IP management etc.
By Anil Kulkarni
Please contact us at info@origiin.com to know more about our services
Subscribe to YouTube Channel HERE
Join India’s largest Linkedin Group: Indian Patent Agent Exam
Nov 5, 2020 | Indian Patents Act 1970
A patent assignment is an act by the patent owner in which the patent owner permanently transfers the patent’s exclusive rights. This transfer of rights is documented in the official patent record. In a patent assignment, the assignee must pay the assignor a consolidated amount and can collect profits from the patented invention subsequently. This qualifies as a consideration.
Patent licensing allows for the creation of value from innovation as well as the advancement of certain other strategic corporate objectives. Bilateral licensing transactions are the hallmark of the ‘traditional’ patent-licensing industry. It is moreover characterized by consequential transaction costs borne by the parties and information asymmetries that threaten to shrink the market over time. An exclusive license encompasses all of the patent’s rights licensee receives except the title. In this instance, the licensee enjoys the same rights as the patent owner, with the exception of the ability to transfer the patent to another individual or company. This restriction exists simply because even though the agreement allows the rights to be transferred; the patent owner retains ownership of the title.
The rights in lieu of the license agreement are predominately granted to the licensee and in accordance to the terms of the said agreement cannot be transferred further. Ergo, patent licensing is only for a limited term, when the license period ends, the owner reclaims his exclusive rights to his invention.
Professor David Teece asserted in an unconventional paper[1] that the ability to construct value from invention was dependent on interrelate assets like marketing, production, and after-sales assistance. Innovators frequently lack direct ownership or control over these assets, forcing them to license out the commercialization process.[2] Licensing can also be used to impact market demand and competitiveness. Patents are licensed out to restrict competitors from further conducting research and development.
This article states the various practices involving the re-assignment clarifying the ownership of the Patent Agreements.
Infringement Litigation
The patentee (licensor) has the sole right to sue for infringement under the Indian patent system.[3] The only statutory exceptions are the exclusive license and the licensee to whom a compulsory license has been granted.[4] A non-exclusive licensee is not allowed to sue for infringement in his or her own name.[5]
Preventing a third party from infringing on the patent, on the other hand, serves the interests of both the licensor and the licensee. Unlicensed usage by a third party will result in ULR fees being charged to the licensor. Similarly, the licensee will be concerned about an infringing competitor who has not been subjected to ULR payments, the expressions of the mutual interest. It may be however become problematic as the expenses of contesting the infringement are more than the patentee’s personal returns, (s)he may not be motivated to file the claim.
Patent buyouts
At least two instances during the early nineteenth century, when both patents and prizes were employed to encourage discovery, Governments integrated the patent and prize systems by purchasing patents. Patent buyouts are appealing because they provide the chance to eliminate monopolistic pricing distortions and duplicate research incentives while increasing rewards for innovative research. It’s crucial to investigate how they implemented the patent buyouts in practice.
Patents scarcely incentivize original research owing to the fact that potential inventors will not consider consumer surplus while deciding whether or not to pursue it. By purchasing the patent for Daguerreotype photography and releasing the technique in the public domain in 1839, the French government blended aspects of the patent system and direct government sponsorship of research. Daguerreotype photography was quickly embraced over the world after the patent was bought out, and it underwent significant technical advances. Patent buyouts like these have the capacity to eradicate monopolistic price distortions and inefficient reverse engineering incentives while further stimulating original research. Determining the price is a major difficulty for any patent buyout mechanism.
The government would propose to buy out patents at this private value times a fixed markup that would roughly cover the gap between the social and private value of inventions. Inventors could have the option of selling or preserving their patents. Government-purchased patents are usually released into the public domain.
However, in order to encourage auction participants to be honest about their appraisals, the government would select a few patents at random and sell them to the highest bidder. Encouragement of invention through such a process would necessitate greater discretion from government officials than the current patent system, but somewhat less discretion than that exercised by the National Institutes of Health.
Patents also restrict research by generating excessive motivation to produce alternatives for patented assets while providing too little incentive to develop complements. Firms can steal rents from existing patent holders by producing replacement inventions. The minimal information available implies that this issue could be intense. Mansfield, Schwartz, and Wagner (1981) discovered that 60 percent of patented discoveries were reproduced within four years, with the average imitation cost being two-thirds of the original cost of development. Potential complementary invention developers, conversely, will have insufficient incentive to create these inventions if they must first invest in developing supplementary inventions before negotiating license arrangements with original patent owners [Green and Scotchmer 1982]. Sometimes, due to asymmetric information, agreements between owners of complementary patents are not achieved, and inventions go underutilized.[6]
Grant Back
Many patent license agreements fail to address licensee improvements, allowing the licensee to file improvement patents of their own, potentially rendering the licensor’s technology obsolete or even preventing the licensor from commercializing its own product with the enhancements. By including “grant back” provisions in license agreements, a licensor can ensure that when licensing out patents covering its technology, any improvements by the licensee are granted back to the licensor. A licensor can ensure that when licensing out patents covering its technology, any enhancements made by the licensee are granted back to the licensor by incorporating “grant back” terms in license agreements.
Literature in relation to Employee-Employer Patent Ownership
By omitting to add a “deemed ownership” provision in the Patents Act of 1970, Indian policymakers missed the mark. Section 39 of the UK Patent Act, Section 132 of the Israeli Patent Act, and Section 6 of the Chinese Patent Act have all codified similar provisions. This deeming theory is founded on the “duty to invent” principle, which states that a person who has a duty to invent cannot have a patent registered in his name. This premise is based on the idea that if an employee has exploited the company’s facilities, technological know-how, or resources, the employer should not be barred from the benefits.
As a corollary, an employee who created the invention during his or her “course and scope of employment” is unable to get a patent in his or her own name. In Darius Rutton Kavasmanek v. Gharda Chemicals, the Bombay High Court was introduced this argument of “duty to invent.” The court, however, refused to evaluate the issue since it was an injunction appeal, and it could not opine on the merits of the case. In addition to the “duty to invent” argument, the “shop-right” principle, which originated in the United States, can be used to address the ownership problem. Regrettably, it has yet to be implemented in India. Even if there is no agreement for royalties, shop-right is a non-exclusive and non-transferable license with the employer to use the innovation without paying royalties. Even if the employee, who is the patent owner, sells his interest in the patent, the employer retains his shop-right in the patent under this doctrine.
When global firms are involved in Research and development activities and their inventors are Indian employees, the above-mentioned flaw in Indian patent law is very troublesome. According to Section 39 of the Patents Act, any resident of India who applies for a patent or causes an application for a patent to be filed in a country outside of India must first obtain authorization from the Controller of Patents.
For instance, a US corporation wishes to submit a patent in the US, but the inventors are Indian employees who live in the country. It might now be argued that the Indian employees, by their patent assignment agreement, have ‘caused’ the patent application to be filed in the United States, necessitating clearance from the Indian Controller of Patents. This is a significant impediment to the employer-company receiving a patent in a timely manner. Such unnecessary delay in an area as dynamic as intellectual property is likely to have an influence on the utilization of resident Indian personnel for invention. Incorporating such a provision that assigns patent ownership to the employer/company, on the other hand, will go a long way toward resolving such issues.
It’s worth noting that the United States Patent Act makes no mention of patent ownership between employers and employees. However, the courts have established a number of precedents that benefit employers“It is feared that if a corporation is denied the advantages of its success, it would cease to subsidize and experiments will go,” the court held in Goodyear Tyres and Rubber Company v. Miller in the United States. In future judgements, Indian courts could take cognizance of this and set better precedents to potentially enable occlude loopholes in the patent law.
With India’s existing patent ownership framework, the employer bears the threat of not owning the invention despite making significant investments. Employers may be hesitant to invest in research possibilities as a result of this. An equivalent approach in India, as in the United States, the United Kingdom, and other nations, would undoubtedly aid in the resolution of patent ownership disputes between employers and employees. If the invention was developed using the employer’s resources and during the course of employment, the employer should be given a say in the patent, even if there is no pre-assignment / assignment agreement for the same involving the abovementioned principles.
There clearly is a dilemma revolving the true ownership of the Patents developed under employment and the legal literature of various countries reflect the very same. The question of ownership, however, in India remains with the employer (with the assignment of intellectual property in the course of employment) development during an employment.
There is a certain exception which that outruns the private benefit and focuses on public good.
Compulsory License
Compulsory license occurs when the government grants authorization to any individual or organization to use, sell, or manufacture a patented design or product for the public good, regardless of the patent owner’s wishes. Compulsory licenses are commonly given in the pharmaceutical industry and in products that meet the standards set forth in Section 84 of the Patent Act 1970. On March 9, 2012, Natco Pharma Ltd. received the first compulsory license in India for making a generic version of Nexavar, a patented Bayer Corporation drug.
Compulsory licensing is provided under Chapter XVI of the Patents Act of 1970 as an essential precaution for defending the public interest. Any interested party can request compulsory license after three years if the invention is not fairly available to the general public. The central government has the authority to file an application with the controller, requesting that the controller endorse a patent with the ‘license of right’.
The provision of the central government was repealed by the amendment. Furthermore, required adjustments were made with regard to whether the public requirements were fulfilled, if the innovation is not manufactured in India or if the patentee refuses to accord a license, by removing a presumption that the public’s requirements are fulfilled based on local manufacture. The amendment also granted the controller the authority to issue a forced license in the event of a national emergency. There is also a provision that allows a third party to apply for a compulsory license even though the invention is not manufactured in India. This shift also allows the controller to revoke the compulsory license if the circumstances that led to it cease to exist.
In simple terms, the choice to assign or license is based on the most profitable commercialization route available to the patent holder. And, while making a decision, the advantages of receiving royalties or alternatively receiving a lump sum price, giving away title, or simply surrendering the rights to commercialize the invention in a certain location for a set length of time must always be evaluated against one another. Assignment may occasionally seem more beneficial than licensing.
Regardless of the fact that the law safeguards a patentee’s interests, the patent holder must prepare an appropriate assignment or license agreement to avoid any potential disputes regarding the ownership. Intellectual property rights have only recently come to the attention of the general public. Where industrial property is adequately protected, which in turn raises the country’s economy, a comprehensive understanding of intellectual property rights is vital. The entire legal infrastructure was furnished by the Government of India. Software, traditional knowledge, plant varieties, and geographical indications have all been accorded specific legal provisions. Among these provisions, certain remedies are only available to the owner of the intellectual property; hence the determination of ownership and proper re-assignment becomes vital.
By: Vinita R. Gaud, Pravin Gandhi College of Law
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join India’s largest Linkedin Group: Innovation & IPR
[1] David J. Teece, “Profiting from Technological Innovation : Implications for Integration, Collaboration, Licensing and Public Policy”, 15 Research Policy 285 (1986).
[2] Ibid. at 296.
[3] The Patents Act, S. 48 (1970)
[4] Id., S.110
[5] Pravin Anand, T. Saukshamaya & Aditya Gupta, India, in Patent Litigation : Jurisdictional Comparisons 201, 203 [Massimo Sterpi et al. (eds.), 2011]; Suchita Saigal, Parul Kumar & Aditya Verma, Licensing Intellectual Property Rights’ Use, in The Law of Business Contracts in India 92, 96 (Sairam Bhat edn., 2009).
[6] The Quarterly Journal of Economics , Nov., 1998, Vol. 113, No. 4 (Nov., 1998), pp. 1137-1167
 In this case of trademark infringement, an exemplary amount of Rs. 1.5 crore was awarded by the Hon’ble Bombay High Court in favour of Glenmark Pharmaceuticals Ltd. (hereinafter referred to as ‘Glenmark’). Galpha Laboratories Ltd. (hereinafter referred to as Galpha) was found to be ‘habitual offender’ for trademark and copyright infringement. What makes this case interesting is, firstly the Galpha’s decision to not contend the suit and secondly the exemplary damages awarded by Hon’ble Bombay High Court. Brief Facts of the case are as follows:
In this case of trademark infringement, an exemplary amount of Rs. 1.5 crore was awarded by the Hon’ble Bombay High Court in favour of Glenmark Pharmaceuticals Ltd. (hereinafter referred to as ‘Glenmark’). Galpha Laboratories Ltd. (hereinafter referred to as Galpha) was found to be ‘habitual offender’ for trademark and copyright infringement. What makes this case interesting is, firstly the Galpha’s decision to not contend the suit and secondly the exemplary damages awarded by Hon’ble Bombay High Court. Brief Facts of the case are as follows:
 Apex laboratories
Apex laboratories A software development agreement is a contract in which one party (the Developer) undertakes to develop software for another party (the Client or the Company). While design and development procedures differ based on the complexity of the project and the team employed, there are a few important issues that are universally applicable and should be taken into account while negotiating the contract.
A software development agreement is a contract in which one party (the Developer) undertakes to develop software for another party (the Client or the Company). While design and development procedures differ based on the complexity of the project and the team employed, there are a few important issues that are universally applicable and should be taken into account while negotiating the contract.

