Jun 11, 2024 | Patent
Claim mapping of patent claims is a technical process used to map one or more claims of a patent to a specific feature, function, component or product featured by the patent to determine if any product or service is allegedly infringing the claims of the patent.
Purpose of Claim Mapping
The primary purpose of a claim map is to determine the occurrence of any form of patent infringement. The patent claim map is generally used during patent infringement analysis, where the claim map provides a clear visualization of all the information analysed in the patent claim.
The claims maps illustrate the infringement of each and every element of the claims of a patent by any existing product or service. The patent claim is infringed when each and every element or component of the patent claim is present in any product or service. It is highly important to interpret the patent claim to determine the patent infringement.
The patent claim mapping process evaluates and identifies the most important patents in a company’s patent portfolio and the level of protection provided by those patents. Claim mapping is highly important during the patent prosecution phase as it is critical to know which claims of the patent need to be retained and which are to be abandoned.
Structure of a Claim map
A claim map is generally a map having a textual and graphical comparison of the claims of a patent and the potentially infringing uses. The claim map may be created by manual examination of the claims, which is time consuming and labour-intensive process. Further, one can use various software tools for examining the claims, which is more efficient and effective than manual examination. The examined claims are used to create the claim map in different forms.
The claim map may be constructed in various forms, such as a map with two columns where the elements of a patent claim and the features of the potentially infringing products or services are mapped along with the related evidence. The claim map may be constructed in three-column forms where the elements of a patent claim, along with supporting citations from the specification of the patent providing interpretations of one or more of the elements of the claim are mapped with the potentially infringing products or services. Further, the claim map can be created in a graphical format where the examined claims are presented on different slides, each focusing on one claim element or a component. The claim maps demonstrate the probable infringement to a patent claim and target to map all the elements of the patent claim to complete the mapping.
Example of a Claim Map

Courtesy: https://www.linkedin.com/pulse/why-claim-charts-underlying-purposes-policies-local-patent-schulman/
Important considerations before mapping the claims
There are several points to be considered before mapping the patent claims. It is important for a claim map to address how the potentially infringing product or service carries out the claim limitation of the patent claim. Also, finding the individual or entity that infringes the patent is critical. In case any wrong individual or entity is accused of infringement, it might lead to unfavorable situations. The claim map must clearly define the infringer along with how or when that infringement has occurred.
Further, it is important not to map inconsequential products as mapping incorrect products may lead to nullifying the claim map. Additionally, while determining the potential infringer and their infringing product or service, care must be taken to map the most significant infringing products or services out of all the infringers. Also, it is critical to include all the evidence in the claim map for a stronger case. Further, it is important to make sure that all relevant evidence present in the claim map is supported by citations.
Conclusion
Claim mapping determines the probability of any form of infringement of a patent by creating a clear visualization of all the similarities between the elements present in a patent claim with potentially infringing products or services. It is important to address a claim’s limitations or requirements in the claim map before concluding that a claim is being infringed. A thoughtfully mapped patent claim map creates valuable intellectual property and facilitates the determination of claims to be retained and abandoned.
Author: Megha S Nadiger, Origiin IP Solutions LLP
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join LinkedIn Group: Innovation & IPR
WhatsApp: +91 74838 06607
Jun 9, 2024 | Contracts, General
Non-Disclosure Agreements (NDA’s) are seen as the backbone of innovation. From business strategies to profit building formulas, they protect confidential information that companies use to expand their business. By offering such protection, NDA’s also provide incentive for innovative research and development which ultimately results in a thriving economy.
Traditionally, to establish the legal legitimacy of an NDA, the use of stamp paper and notarization were seen as essential procedural requirements. They were seen as a way to ensure the authenticity and enforceability of the agreement. Shrimoyee Ghosh, a legal anthropologist in her paper titled, “Not worth the paper it’s written on’: Stamp paper documents and the life of law in India” aptly stated that, “The stamp paper is imbricated in the bureaucratic and legal hubris of viewing writing as a transparent, portable, and durable communicative technology capable of recording and translating fleeting social transactions into taxable and legible evidence of fact and reality.”
However, the rise of electronic agreements and the increasing reliance on the internet have led to debates about the necessity of such traditional methods. In a post pandemic world, where society has recognized the importance of the digital world, the question arises: Should we view these procedures as mandatory requirements anymore?
Challenges with NDA’s In the Digital Age
Today, the registration of a Non-Disclosure Agreement on stamp paper and notarization are not treated as essential procedures to be followed. This is because companies in the digital age favour online signatures (e-signatures) for faster and more convenient agreements. However, there are considerable risks and growing concerns over data security and enforceability of digital NDA’s.
Sharing documents through email, social media platforms and cloud storage creates vulnerabilities. The recent case of ‘Naveen Kumar vs The State of Karnataka (2022)’ exemplifies the risk. The court dealt with a situation where due to insufficient security of data, the confidential information which belonged to the petitioners were stolen by their rival company who proceeded to utilize said information to further their own business. The court acknowledged the increasing number of such cases as a direct result of the digitization of agreements.
The widespread use of e-signatures also raises concern regarding the authentication procedure. The core issue lies in the forgery and tampering of signatures. While these risks also extended to the traditional form of signatures, the process of notarization heavily mitigated the risk. Owing to the fact that digital NDA’s are a recent phenomenon, they lack adequate safeguards. Because of this, cases concerning the forging of e-signatures have grown. In the recent case of Mr Madhukar G Angur vs M/S Alliance Business School (2018)’, the court dealt with a dispute that arose out of a transaction which occurred through an online portal. The plaintiff alleged that there was a forgery of digital signature. Although the Information Technology Act, 2001 outlines steps for the authentication of e-signature, it is evident that we require stronger legal and technological frameworks to protect against the misuse of e-signatures.
Can we overcome these challenges without relying on stamp paper and notarization?
While traditional methods of stamp paper and notarization offer solutions to these authentication concerns, they would have the effect of nullifying digital agreements altogether. However, acknowledging the need for digital agreements, we can argue that the answer lies not in reverting to the past but in developing adequate safeguards for the future of digital agreements.
With regards to the security of confidential information/ data, India has made considerable steps towards securing all forms of digital information. The landmark legislation brought about in 2023 which was ‘The India Digital Personal Data Protection Act’ includes provisions for data security and consequences of any breach which can be adopted in the context of NDA’s. Furthermore, there is considerable merit in the suggestion of using blockchain technology to secure e-signatures. By storing each signature on a tamper proof blockchain ledger and limiting its access it can act as an adequate safeguard.
While stamp paper and notarization have played a significant role, they do not align with the realities of the digital age. The rise of digital NDA’s requires us to move away from these traditional methods. Despite the challenges that arise out of digital NDA’s, we can aim to safeguard confidential information by expanding our legal and technological safeguards rather than relying on procedures from the past.
Author: Ambika Menon, O. P. Jindal Global University
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join LinkedIn Group: Innovation & IPR
WhatsApp: +91 74838 06607
May 12, 2024 | General
Mother’s Day is celebrated to express love, appreciation, and gratitude towards Mothers and Mother figures for their unconditional love, support, and sacrifices. It is not only a time to express gratitude for the nurturing mothers in our lives but also an opportunity to celebrate their remarkable achievements and contributions to the world. While we often acknowledge mothers for their caregiving roles, it’s equally important to recognize their ingenuity and innovative spirit. Throughout history, countless Mothers have made significant contributions to the world of invention, leaving a lasting impact on society. This Mother’s Day, let us delve into the fascinating realm of mother inventors and explore a few of their groundbreaking creations.
The Pioneering Legacy of Mother Inventors
In the vast landscape of invention, certain individuals stand out not only for their groundbreaking creations but also for the barriers they shattered and the paths they forged. Among these pioneers are Mothers whose ingenuity and determination have left an indelible mark on history. From revolutionizing industries to empowering communities, these remarkable women have paved way for innovators and are inspiring generations to come.
From household conveniences to groundbreaking technologies, Mother inventors have played a pivotal role in shaping our daily lives. Despite facing numerous challenges and juggling multiple responsibilities, these remarkable women have demonstrated exceptional creativity and resilience. Their inventions have not only simplified tasks but have also revolutionized industries and sparked new avenues of innovation.
Let us explore a few innovative contributions by Mother Inventors.
- The Disposable Diaper, invented by Marion Donovan
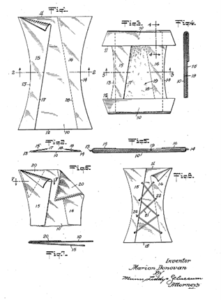 Marion Donovan, a mother of two, revolutionized infant care with her invention of the disposable diaper. In the late 1940s and early 1950s, Donovan, a mother frustrated with the inconvenience of cloth diapers, set out to create a more practical and convenient alternative. Donovan’s first breakthrough came when she developed a waterproof covering for cloth diapers using shower curtains. This innovation helped to prevent leaks and made diaper changes easier. Building on this idea, she then created the first disposable diaper prototype using layers of absorbent material, a waterproof outer layer, and a fastening system to keep the diaper in place. In 1951, Marion Donovan patented her disposable diaper design, known as the “Boater.” The disposable diaper revolutionized childcare practices by providing a more hygienic, convenient, and time-saving option for parents. Since Donovan’s initial invention, disposable diapers have undergone numerous improvements and innovations, including the introduction of elastic leg cuffs, resealable fasteners, and advanced absorbent materials. Today, disposable diapers are a staple product for parents around the world, offering convenience and comfort for both babies and caregivers.
Marion Donovan, a mother of two, revolutionized infant care with her invention of the disposable diaper. In the late 1940s and early 1950s, Donovan, a mother frustrated with the inconvenience of cloth diapers, set out to create a more practical and convenient alternative. Donovan’s first breakthrough came when she developed a waterproof covering for cloth diapers using shower curtains. This innovation helped to prevent leaks and made diaper changes easier. Building on this idea, she then created the first disposable diaper prototype using layers of absorbent material, a waterproof outer layer, and a fastening system to keep the diaper in place. In 1951, Marion Donovan patented her disposable diaper design, known as the “Boater.” The disposable diaper revolutionized childcare practices by providing a more hygienic, convenient, and time-saving option for parents. Since Donovan’s initial invention, disposable diapers have undergone numerous improvements and innovations, including the introduction of elastic leg cuffs, resealable fasteners, and advanced absorbent materials. Today, disposable diapers are a staple product for parents around the world, offering convenience and comfort for both babies and caregivers.
- Glass Bead Liquid Culture Technology, invented by Dr. Seema Prakash.
 Dr. Seema Prakash, a mother of two from Uttar Pradesh, invented Glass Bead Liquid Culture Technology (GBLCT). With Glass Bead Liquid Culture Technology (GBLCT), sterilized glass beads and liquid nutrients are used to replace the agar as the culture medium, where by using the glass beads, the cost of the media is brought down by 94% on a per plant basis. Glass Bead Liquid Culture Technology is different from traditional tissue culture, as traditional tissue culture uses agar as a gel, whereas in Glass Bead Liquid Culture Technology, the media is a liquid that surrounds the glass beads. This allows for aeration when the culture vessels are shaken and the glass beads offer a greater surface area between the piece of plant tissue that is used to initiate the tissue culture and the liquid medium. Using glass beads with liquid beneath them provides good root aeration for the plantlets and when combined with the high humidity in the culture vessels, results in plants that need less hardening because they develop a stronger root system. This invention will help in cost-effective plant cloning, which in turn will benefit Indian Farmers.
Dr. Seema Prakash, a mother of two from Uttar Pradesh, invented Glass Bead Liquid Culture Technology (GBLCT). With Glass Bead Liquid Culture Technology (GBLCT), sterilized glass beads and liquid nutrients are used to replace the agar as the culture medium, where by using the glass beads, the cost of the media is brought down by 94% on a per plant basis. Glass Bead Liquid Culture Technology is different from traditional tissue culture, as traditional tissue culture uses agar as a gel, whereas in Glass Bead Liquid Culture Technology, the media is a liquid that surrounds the glass beads. This allows for aeration when the culture vessels are shaken and the glass beads offer a greater surface area between the piece of plant tissue that is used to initiate the tissue culture and the liquid medium. Using glass beads with liquid beneath them provides good root aeration for the plantlets and when combined with the high humidity in the culture vessels, results in plants that need less hardening because they develop a stronger root system. This invention will help in cost-effective plant cloning, which in turn will benefit Indian Farmers.
- Dishwasher, invented by Josephine Cochrane
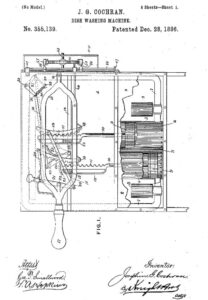
Josephine Cochrane, a mother, invented the first commercially successful dishwasher in the late 19th century. She was frustrated with the chipping and breakage of her fine Chinaware during handwashing, which resulted in designing of a mechanical dishwasher that used high-pressure jets of water to clean dishes. Her invention not only saved time and labour but also preserved delicate tableware, catering to the needs of households and commercial kitchens alike. In 1886, she patented the first practical dishwasher, which used high-pressure jets of water to clean dishes in a wire basket. The invention was showcased at the 1893 World’s Columbian Exposition in Chicago and was initially marketed towards hotels and restaurants. Over time, improvements and innovations have been made to dishwashers, including advancements in efficiency, water and energy consumption, noise reduction, and additional features such as adjustable racks and specialized wash cycles. Now, dishwashers are common household appliances, simplifying the chore of dishwashing for millions of people worldwide.
- Windshield Wipers, invented by Mary Anderson
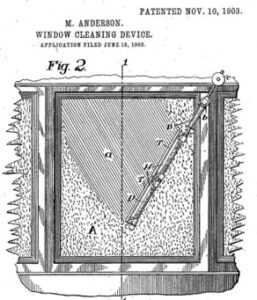 Mary Anderson, a widowed mother and entrepreneur, invented the windshield wiper in 1903. While traveling in New York City, Anderson noticed that streetcar drivers struggled to see through their windows during rainy weather. In search of a solution for this problem, she developed a manually operated windshield wiper system that cleared away rain and snow, improving visibility and safety for drivers. Mary Anderson patented the first operational windshield wiper in 1903, where the invention consisted of a swinging arm mechanism with a rubber blade that could be operated manually from inside the vehicle to clear rain or snow from the windshield. The windshield wiper remains an essential component of vehicle safety, helping drivers maintain clear visibility on the road in adverse weather conditions.
Mary Anderson, a widowed mother and entrepreneur, invented the windshield wiper in 1903. While traveling in New York City, Anderson noticed that streetcar drivers struggled to see through their windows during rainy weather. In search of a solution for this problem, she developed a manually operated windshield wiper system that cleared away rain and snow, improving visibility and safety for drivers. Mary Anderson patented the first operational windshield wiper in 1903, where the invention consisted of a swinging arm mechanism with a rubber blade that could be operated manually from inside the vehicle to clear rain or snow from the windshield. The windshield wiper remains an essential component of vehicle safety, helping drivers maintain clear visibility on the road in adverse weather conditions.
- Kevlar, invented by Stephanie Kwolek
Stephanie Kwolek, a chemist and mother, made a groundbreaking discovery while working at DuPont in the 1960s. In her pursuit of developing a lightweight fibre for tires, Kwolek accidentally created Kevlar, a high-strength synthetic material. Kevlar is a synthetic fiber known for its high tensile strength-to-weight ratio, making it incredibly strong yet lightweight. Kwolek was conducting research on high-performance fibers when she discovered a liquid crystalline solution that could be spun into exceptionally strong fibers. This discovery led to the development of Kevlar, which has since found widespread applications in various industries. Kevlar’s remarkable durability and resistance to impact made it an ideal material for bulletproof vests, revolutionizing personal protective equipment for law enforcement and military personnel worldwide. Kevlar is also used in helmets, gloves, and other protective gear. Additionally, Kevlar is utilized in aerospace applications, such as reinforcing composite materials in aircraft and spacecraft. Its high strength, durability, and heat resistance make it valuable in numerous industrial and consumer products, including tires, ropes, cables, and sporting equipment. Stephanie Kwolek’s invention has had a significant impact on various fields, enhancing safety, performance, and efficiency in numerous applications.
- Home Security System, invented by Marie Van Brittan Brown
 Marie Van Brittan Brown, a nurse and mother, developed the first home security system in 1966. Brown worked long hours as a nurse and often came home late at night. Her husband also worked irregular hours and Brown worried about who might knock on her door if she were home alone at night. Concerned about the safety of her family in their New York City apartment, Brown designed a system comprising peepholes, cameras, and two-way microphones connected to a monitoring device. In 1966, Brown first had the idea, and she soon applied for a patent alongside her husband Albert Brown. The system includes a motorized camera to project images on a monitor with four peepholes. In addition, the invention includes a remote control to unlock doors for easy access to first responders, in emergencies. Brown was later granted an award from the National Scientists Committee for her innovation. The innovative security apparatus laid the groundwork for modern home surveillance systems, empowering homeowners to protect their properties and loved ones.
Marie Van Brittan Brown, a nurse and mother, developed the first home security system in 1966. Brown worked long hours as a nurse and often came home late at night. Her husband also worked irregular hours and Brown worried about who might knock on her door if she were home alone at night. Concerned about the safety of her family in their New York City apartment, Brown designed a system comprising peepholes, cameras, and two-way microphones connected to a monitoring device. In 1966, Brown first had the idea, and she soon applied for a patent alongside her husband Albert Brown. The system includes a motorized camera to project images on a monitor with four peepholes. In addition, the invention includes a remote control to unlock doors for easy access to first responders, in emergencies. Brown was later granted an award from the National Scientists Committee for her innovation. The innovative security apparatus laid the groundwork for modern home surveillance systems, empowering homeowners to protect their properties and loved ones.
Honouring Mother Inventors
As we celebrate Mother’s Day, let’s pay tribute to the remarkable achievements of Mother inventors around the world. Their ingenuity, perseverance, and groundbreaking contributions continue to inspire generations of innovators. From everyday conveniences to life-saving technologies, the legacy of Mother inventors serves as a testament to the power of creativity and determination. So, let us cherish and celebrate the indomitable spirit of motherhood and innovation this Mother’s Day, recognizing the invaluable contributions of mother inventors to our shared human experience.
We wish you all a Happy Mother’s Day!!
Author: Megha Nadiger, Senior Patent Associate, Origiin IP Solutions
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join LinkedIn Group: Innovation & IPR
WhatsApp: +91 74838 06607
May 6, 2024 | Copyright, General
Copyright is a form of intellectual property.[1] Copyright law offers multiple advantages that support in protecting the rights of original creators while also allowing room for creative expression and commentary through the usage.
The Fair Use Doctrine permits the legal copying of a copyrighted work, and it is prescribed under Section 52 of The Copyright Act, 1957[2]. This Doctrine provides an exception against the protections of copyright when the copying of the work is done with the intent and goal of studying, reviewing or even criticising it. As an extension of the same, parody or satire of a copyrighted work is deemed to be covered under this exception. However, from a practical perspective, parody operates in a legal indeterminate area.
Parody and satire are terms used interchangeably, but the two are not identical or equivalent. Although Parody and Satire are both tools of humour that are often used to criticise, review or even merely poke fun at something. However, it is important to note that these two must be necessarily differentiated in order to determine the legality of the same in the context of intellectual property infringement.
While both parody and satire use humor as a tool to effectuate a message, again, the purpose of a parody is to comment on or criticize the work that is the subject of the parody. By definition, a parody is a comedic commentary about a work that requires an imitation of the work. Satire, on the other hand, even when it uses a creative work as the vehicle for the message, offers commentary and criticism about the world, not that specific creative work. Therefore, parodies use copyrighted works for purposes that fair use was designed to protect in the first place.[3] While the difference between parody and satire has been exclaimed on numerous circumstances, the judicial bodies however, tend to club these two terms as the same while passing out their ratio decidendi.
The value of a brand parody
Courts and observers appear to agree that parody has societal significance as critical speech, irrespective to think a particular parody deserves legal protection.
As the Supreme Court has stated, “Like less ostensibly humorous forms of criticism, parody can provide social benefit, by shedding light on an earlier work, and, in the process, creating a new one.” [4]
Although courts generally acknowledge parody, they are noticeably less appreciative of parodies that act as brands.
The First Amendment5 and jurisprudence governing trademark, both grant non-commercial communication a distinct standing. However, the issue with brand parodies goes past doctrine; courts appear to disagree on whether a defendant’s commercial goals conflict with the free speech interest in the parody.
However, there is a compelling argument that, at least in the context of trademarks, the inclusion of parody in a brand achieves expressive purposes that are not at all possible through regular, non-branding speech. Most branding parodies, if not all of them, carry a double-intended message. They are not just making fun of the targeted brand when they use its name.
Beyond just doing a parody for some trademark, brand parodies also capture the branding mechanism and use it to provoke the critical thought about the atmosphere of brands in our society.
Therefore, brand parody that are genuine in nature have societal value and are not likely to lead to confusion or a loss of the uniqueness of the target mark; as a result, trademark courts should rarely forbid them. however, disputes over brand parodies can go on for years as courts disagree on how and when the law should apply in that circumstance.
Because of the commercial nature of these parodies or because of the impression of exploitation and clout riding that has been created, there may be ambiguity and incorrect application of the law.
Circumstances when parody cannot be used as a defence
While parody may be protected under the fair use doctrine in some cases, it is not an absolute defence, and each situation should be evaluated on the basis of the case and legal jurisdiction in order to determine its validity. Even while there is no existence of an absolute defence for parody, common sense and rationality suggests that it may be difficult to prove what does and does not amount to a ‘successful parody’, which could be where the idea of humour is directly communicated to the target audience. Hence, proving infringement against a strongly- alleged claim of parody is likely to be very challenging. There are instances where parody is not subject to blanket protection under the doctrine of fair use provided by the statute.
In the case of Leibovitz v. Paramount Pictures Corporation[5], an acclaimed photographer by the name Leibovitz had taken a picture of actress Demi Moore during her pregnancy during a professional photoshoot. She was seen to be making passionate facial expressions in the picture. Certain aspects of the picture stood out, such as the way the subject was positioned and posed, the kind of lightning, etc. that gave a distinct feel for the same. The controversy now, which led to the case, stemmed from the fact that Paramount Pictures released a picture of actress Leslie Nielsen shortly after the Leibovitz photo, suggesting ideas that were similar to those in Demi Moore’s image.
The stance taken by the court made the concerned parties realize that anything under the sun does not become a parody simply because it involves a trace of humor. If it is discovered that the defendant has tried to take advantage of a reputed work only to make commercial gains by simply involving some humor in the work, then such a work can be denied the defence of parody.
Similarly, in the famous Superman Logo case of 2015[6], in the United States of America, The defendant was an apparel line that featured the well-established and recognisable Superman shield design on its items. The word “Dad” was shaped like a superman emblem on the T-shirts. The defendant said that there was little chance that customers would confuse their SuperDad T-Shirts for the iconic Superman insignia because they were a blatant and obvious mimic of it.
The defendants of this particular dispute had the contentions that the word ‘dad’ relates to the acknowledgment of the real-life hero which a father is to their children and the undue self-importance that is given. While this was a creative argument stirred up by the Counsel for defendants, it was not enough for convincing the honourable court. The court dismissed the contentions laid down by the defendants and held that although the defendant’s use of the word ‘Dad’ was with the intent of humour, the purpose is only to promote their t-shirts using the famous logo of the plaintiff, and this certainly cannot be protected as a parody under the law.
Conclusion
In conclusion, the Fair Use Doctrine and copyright laws offer an essential balance between defending the rights of the original creators and permitting parody as a form of creative expression and commentary. Parodies can be regarded as a tool for critical discourse since they provide new light on past works and generate new points of view when the intention is present. However, the legal landscape for brand parodies is complex, as courts grapple with the intersection of commercial interests and free speech rights. While genuine brand parodies have societal value, disputes can be prolonged and contentious. Parody is not an absolute defense, and its validity depends on the specific circumstances and legal jurisdiction for which the dispute can be handled in the interests of justice, equity and good conscious
Author: Ayushman Kumar B.
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join LinkedIn Group: Innovation & IPR
WhatsApp: +91 74838 06607
Apr 29, 2024 | General
“You are an original when you stay true to what you know.” Following this philosophy for 40 years since 1984, Natural Ice Cream has become a well-known brand for using fruits in creating ice creams. With around 125 flavors to offer 147 outlets across the country and the recently launched factory in Mumbai, Natural Ice Cream is poised to continue expanding its reach in spreading the joy of fruits and natural ingredients in the form of delicious and mouth-melting ice creams.
Where today, most ice creams in the market consist of fruit flavors, stabilizers, and additives; what makes Natural Ice cream so different from others is being true to their tradition and traditional methods, which include the non-usage of any preservatives, stabilizers and artificial colors or chemicals and relying on three ingredients only, i.e., milk, fruit, and sugar, most of them sourced from India, making Natural Ice cream delicious and wholesome.
Furthermore, Natural Ice Cream is recognized as one of the pioneering brands responsible for introducing flavors such as Tender Coconut Ice Cream, Jackfruit Ice Cream, Watermelon Ice Cream, and Chickoo Ice Cream, among others, which are consistently available throughout the year. They also pioneered the idea of seasonal specials like Naturals Sitaphal Ice Cream, Naturals Mango Ice Cream, Pink Guava, Lychee, and more. Additionally, several new flavor concepts, including festive flavors, Friday Funday flavors, and berry festival flavors, were introduced a few years later, all offered at highly affordable prices.
Consistent focus on evolving while preserving its authentic flavors drives Natural Ice Cream’s sustained growth. Unsurprisingly, the brand has been recognized among the top 10 Home-grown brands in India by the KPMG Consumer survey and honored as Icons of India by the Economic Times.
History
The journey began with a fruit venture in a small village in Mangalore, where Mr. Raghunandan Kamath, the founder, aided his father, a mango seller. Enchanted by the realm of fruits, he mastered the skills of selecting, plucking, sorting, and preserving ripe produce. This profound knowledge became an asset for the company in crafting fruit ice creams.
From Mangalore to Bombay, armed with a second-class ticket and a brilliant idea, Mr. Raghunandan Kamath embarked on his entrepreneurial journey. Initially serving Pav Bhaji as the main dish, he cleverly added ice cream as an enticing addition to attract customers. This modest eatery quickly garnered attention, evolving into a bustling ice cream parlor known as “NATURALs,” inaugurated as the First Outlet in Juhu Scheme, Mumbai, on February 14, 1984. The overwhelming demand for their ice cream led to traffic congestion in Juhu’s narrow lanes. To meet the increasing demand, Mr. R S. Kamath had to innovate and develop new machines capable of producing more ice cream using existing methods.
By 1994, the company expanded its presence by inaugurating five additional outlets in Malad, Borivali, Bandra, Lokhandwala Complex, and Vile Parle. In 2000, Pune became the company’s first branch outside of Mumbai, further expanding into Ahmedabad, Goa, Hyderabad, Bangalore, Jaipur, Delhi, and Kolkata. In response to its increasing popularity, the company also introduced distinctive thermocol packaging, quickly becoming ubiquitous nationwide.
Trademark
One of the obstacles that Natural Ice Cream faced was its trademark. When Mr. Raghunandan Kamath began his business of a full-fledged ice cream parlor in Mumbai on February 14, 1984, under the mark ‘NATURAL’, it became the company’s identity. Over time, the mark obtained trademark registrations across multiple classes, including 29, 30, 35, 42, and 43. Under this trademark, the company has expanded its network to 140 franchisees across 42 cities in India. Moreover, it has extensively advertised on social media platforms like Facebook and Twitter. Despite having such a reputation, there were instances where the company had to deal with consumer confusion due to the similarity of trademarks with those of other companies.
One such instance can be found in the recent case of Siddhant Icecreams LLP & Ors. Vs. Ameet Pahilani & Ors, where the plaintiff filed a suit against Partners of NIC Natural Ice Creams seeking a decree of permanent injunction from trademark infringement along with an application for ad-interim injunction before the Hon’ble Delhi High Court[1]. It occurred when NIC NATURAL ICE CREAMS, another ice cream firm, started enticing customers with its varied range of ice cream flavors, which were not only accessible in physical stores throughout India but also featured on online food delivery platforms, much like Natural Ice Cream. However, since both firms utilized the same mark, ‘NATURAL,’ in an equal shade of green and dealt primarily with comparable products and services, as well as similar packaging, concerns of infringement, misrepresentation, and customer confusion were likely to arise. The primary allegation was that Ameet Pahilani, a partner at NIC Natural Ice Creams, was formerly associated with one of the franchisees of Natural Ice Cream. Following the termination of their agreement, he allegedly registered a mark bearing the name ‘NIC Natural Ice Creams’ in a dishonest manner.
In conclusion, on October 19, 2022, Justice Jyoti Singh issued an “ex parte ad interim injunction” in favor of the plaintiffs Siddhant Ice Creams LLP & others. The court found that all the evidence and facts favor the plaintiff, and thus, not granting an injunction order could result in “irreparable loss” to the plaintiffs. The court’s ruling forbade the defendants, NIC Natural Ice Creams, from using the plaintiffs’ marks in any way, including marks that were similar to them, and from utilizing the same trade dress, colors, and packaging. Additionally, they were barred from using domain names containing the mark ‘NATURAL’ and the plaintiffs’ marks as meta-tags or keywords for advertisement. The court even ordered food delivery aggregators to verify that infringing trademarks were not used in conjunction with the defendants’ business[2]. An appeal by the defendants followed this injunction order. However, both parties reached an interim arrangement on November 25, 2022, allowing them to conduct business without prejudice.
Before this incident, Natural Ice Cream faced a similar situation in the case Siddhant Icecreams LLP and Ors v. Natural Ice Cream And Anr., [3]in which Raghunandan Kamath promoted firms Siddhant Icecreams LLP, and Kamaths Ourtimes Ice Creams Pvt Ltd filed a lawsuit against the Manjalpur (Vadodara)-based ‘Natural Ice Cream.’ In addition to requesting damages for trademark infringement of Rs. 150 crore, the lawsuit aimed to stop the defendant brand in Vadodara from using the same trade name for its goods. The court ruled that the proprietor of the defendant company had no independent right to use the ‘NATURAL’ mark, even if the signage indicated usage ‘since 1992’. The plaintiffs, registered proprietors with usage dating back to 1984, sufficiently demonstrated a prima facie case. This led to the injunction order restraining the defendants from using or infringing the ‘Natural’ Family registered marks for their products or business names until September 9. Due to such brand recognition, Natural Ice Cream has been able to preserve and protect its brand identity and maintain consumer trust.
Conclusion:
In conclusion, Natural Ice Cream has garnered numerous accolades over the years, including the Corporation Bank’s National SME’s Excellence Award in the Food and Agro-Industry in 2006, Best in Customer Service – Regional Retailer Of the Year in 2013, a gold medal for the most innovative ice cream flavor (cucumber) in the Great Indian Ice Cream Contest in 2014, and recognition in 2016 for its homegrown concept in food service by Coca-Cola Golden Spoon Awards. These achievements underscore the brand’s reputation, which extends beyond mere advertising to the genuine appreciation of its customers. With a keen understanding of fruit-based ice cream’s potential, the company and its founder have consistently delivered a delicious end product celebrated nationwide. Natural Ice Cream remains a symbol of excellence in the ice cream industry, firmly establishing itself as one of India’s premier homegrown brands.
Author: Gautam Bhatia, CHRIST University
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join LinkedIn Group: Innovation & IPR
WhatsApp: +91 74838 06607


 Marion Donovan, a mother of two, revolutionized infant care with her invention of the disposable diaper. In the late 1940s and early 1950s, Donovan, a mother frustrated with the inconvenience of cloth diapers, set out to create a more practical and convenient alternative. Donovan’s first breakthrough came when she developed a waterproof covering for cloth diapers using shower curtains. This innovation helped to prevent leaks and made diaper changes easier. Building on this idea, she then created the first disposable diaper prototype using layers of absorbent material, a waterproof outer layer, and a fastening system to keep the diaper in place. In 1951, Marion Donovan patented her disposable diaper design, known as the “Boater.” The disposable diaper revolutionized childcare practices by providing a more hygienic, convenient, and time-saving option for parents. Since Donovan’s initial invention, disposable diapers have undergone numerous improvements and innovations, including the introduction of elastic leg cuffs, resealable fasteners, and advanced absorbent materials. Today, disposable diapers are a staple product for parents around the world, offering convenience and comfort for both babies and caregivers.
Marion Donovan, a mother of two, revolutionized infant care with her invention of the disposable diaper. In the late 1940s and early 1950s, Donovan, a mother frustrated with the inconvenience of cloth diapers, set out to create a more practical and convenient alternative. Donovan’s first breakthrough came when she developed a waterproof covering for cloth diapers using shower curtains. This innovation helped to prevent leaks and made diaper changes easier. Building on this idea, she then created the first disposable diaper prototype using layers of absorbent material, a waterproof outer layer, and a fastening system to keep the diaper in place. In 1951, Marion Donovan patented her disposable diaper design, known as the “Boater.” The disposable diaper revolutionized childcare practices by providing a more hygienic, convenient, and time-saving option for parents. Since Donovan’s initial invention, disposable diapers have undergone numerous improvements and innovations, including the introduction of elastic leg cuffs, resealable fasteners, and advanced absorbent materials. Today, disposable diapers are a staple product for parents around the world, offering convenience and comfort for both babies and caregivers. Dr. Seema Prakash, a mother of two from Uttar Pradesh, invented Glass Bead Liquid Culture Technology (GBLCT). With Glass Bead Liquid Culture Technology (GBLCT), sterilized glass beads and liquid nutrients are used to replace the agar as the culture medium, where by using the glass beads, the cost of the media is brought down by 94% on a per plant basis. Glass Bead Liquid Culture Technology is different from traditional tissue culture, as traditional tissue culture uses agar as a gel, whereas in Glass Bead Liquid Culture Technology, the media is a liquid that surrounds the glass beads. This allows for aeration when the culture vessels are shaken and the glass beads offer a greater surface area between the piece of plant tissue that is used to initiate the tissue culture and the liquid medium. Using glass beads with liquid beneath them provides good root aeration for the plantlets and when combined with the high humidity in the culture vessels, results in plants that need less hardening because they develop a stronger root system. This invention will help in cost-effective plant cloning, which in turn will benefit Indian Farmers.
Dr. Seema Prakash, a mother of two from Uttar Pradesh, invented Glass Bead Liquid Culture Technology (GBLCT). With Glass Bead Liquid Culture Technology (GBLCT), sterilized glass beads and liquid nutrients are used to replace the agar as the culture medium, where by using the glass beads, the cost of the media is brought down by 94% on a per plant basis. Glass Bead Liquid Culture Technology is different from traditional tissue culture, as traditional tissue culture uses agar as a gel, whereas in Glass Bead Liquid Culture Technology, the media is a liquid that surrounds the glass beads. This allows for aeration when the culture vessels are shaken and the glass beads offer a greater surface area between the piece of plant tissue that is used to initiate the tissue culture and the liquid medium. Using glass beads with liquid beneath them provides good root aeration for the plantlets and when combined with the high humidity in the culture vessels, results in plants that need less hardening because they develop a stronger root system. This invention will help in cost-effective plant cloning, which in turn will benefit Indian Farmers. Mary Anderson, a widowed mother and entrepreneur, invented the windshield wiper in 1903. While traveling in New York City, Anderson noticed that streetcar drivers struggled to see through their windows during rainy weather. In search of a solution for this problem, she developed a manually operated windshield wiper system that cleared away rain and snow, improving visibility and safety for drivers. Mary Anderson patented the first operational windshield wiper in 1903, where the invention consisted of a swinging arm mechanism with a rubber blade that could be operated manually from inside the vehicle to clear rain or snow from the windshield. The windshield wiper remains an essential component of vehicle safety, helping drivers maintain clear visibility on the road in adverse weather conditions.
Mary Anderson, a widowed mother and entrepreneur, invented the windshield wiper in 1903. While traveling in New York City, Anderson noticed that streetcar drivers struggled to see through their windows during rainy weather. In search of a solution for this problem, she developed a manually operated windshield wiper system that cleared away rain and snow, improving visibility and safety for drivers. Mary Anderson patented the first operational windshield wiper in 1903, where the invention consisted of a swinging arm mechanism with a rubber blade that could be operated manually from inside the vehicle to clear rain or snow from the windshield. The windshield wiper remains an essential component of vehicle safety, helping drivers maintain clear visibility on the road in adverse weather conditions. Marie Van Brittan Brown, a nurse and mother, developed the first home security system in 1966. Brown worked long hours as a nurse and often came home late at night. Her husband also worked irregular hours and Brown worried about who might knock on her door if she were home alone at night. Concerned about the safety of her family in their New York City apartment, Brown designed a system comprising peepholes, cameras, and two-way microphones connected to a monitoring device. In 1966, Brown first had the idea, and she soon applied for a patent alongside her husband Albert Brown. The system includes a motorized camera to project images on a monitor with four peepholes. In addition, the invention includes a remote control to unlock doors for easy access to first responders, in emergencies. Brown was later granted an award from the National Scientists Committee for her innovation. The innovative security apparatus laid the groundwork for modern home surveillance systems, empowering homeowners to protect their properties and loved ones.
Marie Van Brittan Brown, a nurse and mother, developed the first home security system in 1966. Brown worked long hours as a nurse and often came home late at night. Her husband also worked irregular hours and Brown worried about who might knock on her door if she were home alone at night. Concerned about the safety of her family in their New York City apartment, Brown designed a system comprising peepholes, cameras, and two-way microphones connected to a monitoring device. In 1966, Brown first had the idea, and she soon applied for a patent alongside her husband Albert Brown. The system includes a motorized camera to project images on a monitor with four peepholes. In addition, the invention includes a remote control to unlock doors for easy access to first responders, in emergencies. Brown was later granted an award from the National Scientists Committee for her innovation. The innovative security apparatus laid the groundwork for modern home surveillance systems, empowering homeowners to protect their properties and loved ones.

