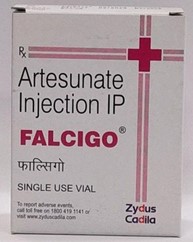
 In this case, the two companies i.e., Cadila Healthcare and Cadila Pharmaceuticals had taken over the business of Cadila Group and one of the conditions of such takeover of Cadila Group was that, both the companies got equivalent right to use the name ‘CADILA’. The suit was brought before the court by Cadila Healthcare when it came to their knowledge that Cadila Pharmaceutical is using the mark “FALCITAB” which is similar to their mark “FALCIGO”; and that the mark was applied by Cadila Pharmaceutical for similar drug.
In this case, the two companies i.e., Cadila Healthcare and Cadila Pharmaceuticals had taken over the business of Cadila Group and one of the conditions of such takeover of Cadila Group was that, both the companies got equivalent right to use the name ‘CADILA’. The suit was brought before the court by Cadila Healthcare when it came to their knowledge that Cadila Pharmaceutical is using the mark “FALCITAB” which is similar to their mark “FALCIGO”; and that the mark was applied by Cadila Pharmaceutical for similar drug.
Hence, though this suit Cadila Healthcare claimed for injunction against Cadila Pharmaceutical to restrain them from using a mark which is deceptively similar and is likely to cause confusion. Brief facts of the case are as follows:
- Cadila Healthcare manufactured the drug “FALCIGO” which was used for treatment of cerebral malaria known as ‘Falcipharum’. They applied for Trade Mark registration in class 5 and obtained permission of Drug Controller General (India) to market the drug on 20th August, 1996 and 7th October, 1996 respectively, and have been manufacturing and selling their drug throughout India under the mark “FALCIGO” post such approvals.
- Cadila Pharmaceutical got permission on 10th April, 1997 from Drug Controller General (India) to manufacture drug containing “Mefloquine Hydrochloride” which was also used for treatment of ‘Falcipharum’. They manufactured and sold the said drug under the mark “FALCITAB”
- For the use of same, civil suit for injunction was filed by the Cadila Healthcare claiming that Cadila Pharmaceutical is passing-off their product under the pretext of same drug for treatment of similar disease and the same is likely to cause confusion.
Arguments
By Cadila Healthcare i.e., Appellant
- It was contended by Cadila Healthcare that similar mark had been used by Cadila Pharmaceutical for treatment of similar disease which is likely to cause confusion and deception.
- That the contention of Cadila Pharmaceutical that the mark is used in ‘Schedule L’ drug which is sold only to hospitals and clinics hence there is no chance of confusion does not hold true as chances human error and confusion cannot be ruled out even if it is handled by trained medical practitioner.
- That Cadila Pharmaceutical is passing off drug of Cadila Healthcare by using the mark “FALCITAB” for another similar drug.
By Cadila Pharmaceutical i.e., Respondent
- Cadila Pharmaceutical contented that the prefix “FALCI” of their mark “FALCITAB” was taken from the disease ‘Falcipharum’, and it is common practice in pharmaceutical industry to name a drug after the disease it is claiming to cure.
- They also contended that the said drug in dispute is ‘Schedule L’ drug and not ‘Schedule H’ drug, which means that the drug would not be sold in retail chemist shop, it will only be sold to hospitals and clinics. As a result, there could not be remote chance of confusion and deception.
Issue
The core issue involved in this case was whether the mark of Cadila Pharmaceutical i.e., “FALCITAB” is similar to the mark of Cadila Healthcare i.e., “FALCIGO”?
Proceedings
Before Trial Court
The injunction application of Cadila Healthcare was dismissed by The Extra Assistance Judge, Vadodara. It was observed that the two drugs “FALCIGO” and “FALCITAB” differed in appearance and formulation, and they can only be sold to hospitals and clinics which diminishes the chance of confusion and deception compared to when these are to be sold to individuals.
Before High Court
An appeal was preferred before High Court against the decision of Trial Court, Hon’ble High Court affirmed with the decision of Trial Court and after examining various aspects held that the likelihood of confusion caused to an unwary consumer is not there and there is little chance of passing off.
Before Supreme Court
At the forefront Hon’ble Supreme Court said that, through this judgement it does not tend to interfere with the decision and findings of lower court, the judgement is passed only to set out principles which are to be kept in mind while dealing with issue of passing off especially in medical products.
Hon’ble Supreme Court examined in detail various precedents of domestic and foreign jurisdictions, and it was observed by Supreme Court that howsoever detailed and minute the foreign precedents are, the same could not be made applicable to India where there is no common language, where large percentage of people are not literate and only few people know English language. Purchasers of India are to be kept in mind before any pronouncement as the confusion of the identity of the product itself could have serious effects on the public health.
It was further observed by court that even though the drugs in question are ‘Schedule L’ drugs which are sold only to hospitals and clinics, it does not rule out the possibility of confusion among professionals dispensing medicines.
And lastly, Hon’ble Supreme Court laid out various factors to be considered in case of passing off, for deciding question of deceptive similarity which are as follows:
- “The nature of the marks i.e., whether the marks are word marks or label marks or composite marks, i.e., both words and label works.
- The degree of resemblances between the marks, phonetically similar and hence similar in idea.
- The nature of the goods in respect of which they are used as trademarks.
- The similarity in the nature, character and performance of the goods of the rival traders.
- The class of purchasers who are likely to buy the goods bearing the marks they require; on their education and intelligence and a degree of care they are likely to exercise in purchasing and/or using the goods.
- The mode of purchasing the goods or placing orders for the goods; and
- Any other surrounding circumstances which may be relevant in the extent of dissimilarity between the competing marks”.
Conclusion
Through this judgement, Supreme Court gave a meticulous interpretation of various precedents and laid out factors to be considered while dealing with infringement or passing off suits, especially when it involves medicinal products. It was laid out by the court that before granting permission to manufacture a drug, the applicant has to satisfy Drug Controller General that there will be no confusion or deception in market by using a certain brand name. Though Hon’ble Supreme Court did not interfere with the decision of the lower court and gave them directions for expeditious disposal of suit, it however laid down the principles and factors which are to be kept in mind while adjudicating upon passing off suits and for deciding question of deceptive similarity.
Dhruv Dangayach, Ramaiah College of Law
Please contact us at info@origiin.com to know more about our services (Patent, Trademark, Copyright, Contract, IP Licensing, M&A of companies)
Subscribe to YouTube Channel HERE
Join Linkedin Group: Innovation & IPR
Whatsapp: +91 74838 06607



